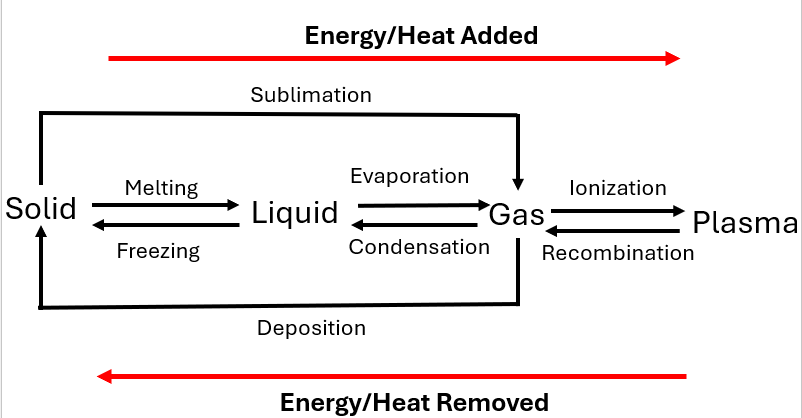
Melting
Melting is the process in which a solid turns into a liquid when it absorbs heat, causing its molecules to gain enough energy to overcome the forces holding them together. This occurs at a specific temperature called the melting point.
Freezing
Freezing is the opposite of melting, where a liquid turns into a solid as it loses heat. The molecules in the liquid slow down and arrange themselves into a rigid structure, which occurs at the freezing point.
Evaporation
Evaporation is the process where a liquid turns into a gas at its surface, typically at temperatures below its boiling point. Molecules at the surface of the liquid gain enough energy to escape into the air.
Condensation
Condensation is the process in which a gas turns into a liquid when it loses heat. As the gas cools, its molecules slow down and bond together, forming droplets of liquid.
Ionization
Ionization is the process by which an atom or molecule gains or loses an electron, becoming a charged ion. This can occur through energy absorption, such as through heat or radiation.
Recombination
Recombination is the process where a free electron and an ion come together to form a neutral atom or molecule. This typically happens when a gas cools and the electron is captured by a positively charged ion.
Sublimation
Sublimation is the transition of a substance from a solid directly to a gas without passing through the liquid phase. It occurs when molecules in the solid gain enough energy to break free and enter the gas phase.
Deposition
Deposition is the process where a gas turns directly into a solid without first becoming a liquid. This happens when gas molecules lose enough energy for them to bond together into a solid form.
